By: Mark M. Fukuda, MD (COL, USA); Mariusz Wojnarski, MD (MAJ, USA); Nicholas Martin, PhD (LCDR, USN); Victor Zottig, PhD (MAJ, USA); Norman C. Waters, PhD (COL, USA)
Editorial
For decades, malaria infections acquired in Korea have posed a significant threat to both Korean military and civilians and to U.S. Department of Defense (DOD) personnel. In Korea, malaria infections are caused exclusively by the species Plasmodium vivax (PV). Despite the use of chloroquine chemoprophylaxis during the Korean War (1950–1953), thousands of cases of PV malaria were diagnosed in Korea among U.S. military personnel.1 As troops returned home, however, many more cases were diagnosed stateside, inundating military hospitals and leading to research on the use of primaquine to treat what were termed "late attacks of Plasmodium vivax of Korean origin".2
Since the Korean War, the Republic of Korea (ROK) has made significant strides in controlling the disease, chiefly through an aggressive eradication program in the 1970s. So successful was the program that the World Health Organization declared the ROK malaria free in 1979.3 However, in 1993, ROK experienced a PV malaria resurgence that reached a peak of 1,600 cases in 19974 before gradually tapering, with ROK experiencing only 601 cases in 2016.5
In this issue of the MSMR, Klein et al. report a cluster of 11 U.S. soldiers with PV.6 The cases were likely acquired at Dagmar North training area located near the southern border of the demilitarized zone (DMZ). As was true during the Korean War, more cases (n=9, 82%) in the cluster were diagnosed long after redeployment to the U.S., an estimated 8–11 months after presumed exposure to the mosquitoes that transmitted the infections. Due to recent historically low numbers of PV malaria cases among DOD personnel in the ROK, and no mortality due to PV, chemoprophylaxis is not routinely administered to service members in the ROK, except among Marine Corps personnel when training near the DMZ (Surgeon Office, U.S. Marine Corps Forces, Pacific; personal communication Oct. 25, 2018).
The most relevant contemporary report of malaria risk in the ROK was published in 2016.7 In this study, mosquitoes were collected at ROK installations near the DMZ, speciated, assessed for PV infection, and correlated with human PV cases. The report concluded the following: 1) that the mosquito species Anopheles kleini was likely the main culprit vector responsible for PV transmission in the ROK; 2) population densities of PV-infected mosquitoes were highest in ROK installations closer to the DMZ; and 3) PV mosquito infection rates correlated highly with the number of PV cases in ROK Army soldiers.
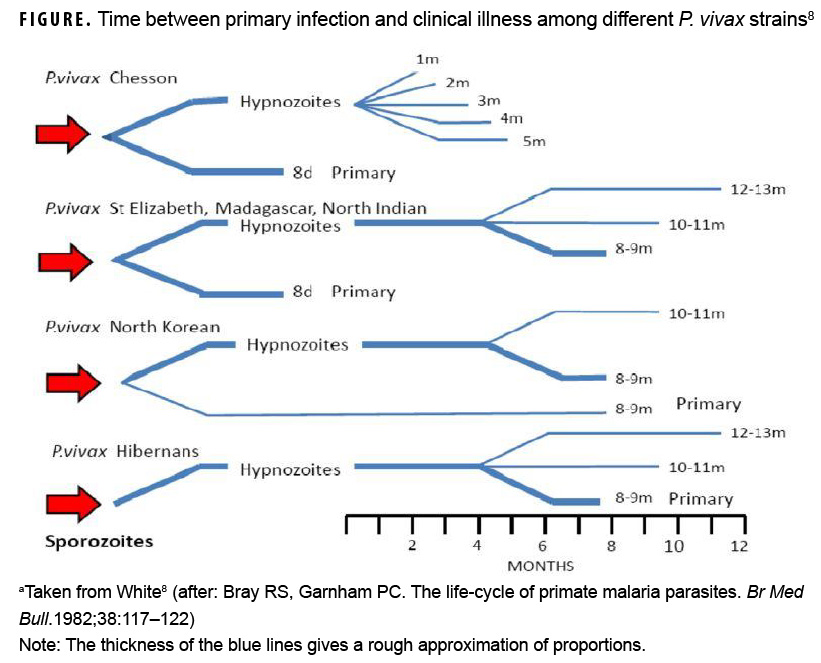
From a chemoprophylaxis perspective, it is instructive to consider the peculiar biology of Korean PV malaria. Korean PV strains are classified as "temperate zone" and are unique in that as many as 40–50% of infected individuals may not manifest the symptoms of their primary illness until 6–11 months after infection.4,8 The Figure shows that time between primary infection and clinical illness among different PV strains ranges between 8 days and 8–13 months.8 Temperate zone PV biology reflects a possible evolutionary adaptation that enables these strains to remain latent as hypnozoites (i.e., "sleeping" parasites) through the cool or cold months that are inhospitable to mosquito vectors in temperate climates.8 As in the postKorean War period, late attacks of Plasmodium vivax of Korean origin continue to be observed. A 2007 review of malaria outbreaks in U.S. military personnel described 74 ROK-acquired PV malaria cases and an estimated 45% were diagnosed more than 240 days after the mid-points of their last ROK exposure period.9 Because U.S. military personnel rotate frequently in and out of the ROK, PV biology virtually ensures that a significant number of DOD personnel with ROK-acquired infections will not become symptomatic until their next duty station—whether in the U.S. or at another location outside of the contiguous U.S.
PV latency and entomological studies indicate that the following should be considered in anticipation of further exposure of DOD personnel in Korea. First, scientific reports unquestionably point to proximity to the DMZ as the highest PV risk factor, while few cases are diagnosed south of Seoul.6 It is impossible to determine how much of this risk is due to a specific ecology within the DMZ or to the presence of a high PV case burden in the nearby Democratic People's Republic of Korea. Thus, continued emphasis on surveillance of Anopheles spp. and identification to species is warranted to identify the geographical and seasonal impact on malaria transmission in the ROK. Secondly, command emphasis on personal protective measures such as use of insecticide treated uniforms and impregnated bednets and on education about malaria risk and prevention is highly warranted. Lastly, because latent cases likely constitute the majority of cases acquired in the ROK, chemoprophylaxis practice should take into account both recent data on acute infections diagnosed shortly after exposure as well as latent infections presenting months thereafter. In this regard, analysis and dissemination of centralized DOD malaria data, such as in the annual MSMR issue,10 are critically important to inform DOD Force Health Protection malaria practice.
The possibility of latent PV infection and frequent deployment tempo warrant consideration of the role of chemoprophylaxis, particularly with respect to "terminal prophylaxis" mdash;a term referring to the pre-emptive treatment of hypnozoites to prevent latent or relapsing malaria. A major challenge for terminal prophylaxis is compliance with up to 14 days of daily dosing with primaquine, until recently the only drug capable of killing hypnozites. For example, a survey of U.S. Army Rangers returning from Afghanistan found that self-reported compliance rates were 52% for weekly chemoprophylaxis, 41% for terminal (post-deployment) chemoprophylaxis, and 31% for both weekly and terminal chemoprophylaxis.11
In Aug. 2018, tafenoquine, an oral long-acting primaquine analogue was approved by the U.S. Food and Drug Administration for prophylaxis against all malaria species for up to 6 months' duration. Tafenoquine, (Arakoda™), was originally discovered by scientists at the Walter Reed Army Institute of Research (WRAIR) and further developed for a prophylaxis indication at the U.S. Army Medical Materiel Development Activity. Critically, tafenoquine/Arakoda™ confers a major advantage over primaquine because of its requirement for only weekly "maintenance" dosing during exposure in malaria endemic areas in contrast to the daily dosing requirement for doxycycline or Malarone®. Significantly, tafenoquine also includes an indication for terminal prophylaxis consisting of a single dose given 7 days after the last maintenance dose upon leaving the malaria endemic area.12 These dosing options provide commanders with the option to more feasibly monitor dosing by directly observed therapy. The implications of the "real world" effectiveness brought by this soldier/commander-friendly dosing option, whether employed to prevent latent P. vivax cases—or infections with other species—are a welcome advance in the fight against malaria.
Author affiliations: Department of Bacterial and Parasitic Disease, U.S. Army Directorate, Armed Forces Research Institute of Medical Science, Bangkok, Thailand (COL Fukuda, MAJ Wojnarski, LCDR Martin, COL Waters); U.S. Army Medical Materiel Development Activity, Fort Detrick, MD, (MAJ Zottig).
Disclaimer: Material has been reviewed by the Walter Reed Army Institute of Research and there is no objection to its publication. The opinions or assertions contained herein are the views of the authors and do not necessarily reflect the official policy or position of the Army, the Department of Defense, or the U.S. Government.
References
- Long AP. General Aspects of Preventive Medicine in the Far East Command. Recent Advances in Medicine and Surgery Based on Professional Medical Experiences in Japan and Korea. Vol II. Washington, D.C.: U.S. Army Medical Service Graduate School; 1954:248-269.
- Jones R Jr, Jackson LS, Di Lorenzo A, et al. Korean vivax malaria. III. Curative effect and toxicity of primaquine in doses from 10 to 30 mg. daily. Am J Trop Med Hyg.1953;2(6):977–982.
- World Health Organization. Synopsis of the World Malaria Situation, 1979. Wkly Epidem Rec. 1981;56(19):145–152.
- Feighner BH, Pak SI, Novakoski WL, Kelsey LL, Strickman D. Reemergence of Plasmodium vivax malaria in the Republic of Korea. Emerg Infect Dis. 1998;4(2):295–297.
- Global Malaria Programme. World Malaria Report 2017. Geneva: World Health Organization.
- Klein TA, Seyoum B, Forshey BM, et al. Cluster of vivax malaria in U.S. Soldiers training near the demilitarized zone, Republic of Korea during 2015. MSMR. 2018;25(11):4–9.
- Chang KS, Yoo DH, Ju YR, et al. Distribution of malaria vectors and incidence of vivax malaria at Korean army installations near the demilitarized zone, Republic of Korea. Malar J. 2016;15(1)259.
- White, NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011 (10):297.
- Ciminera P, Brundage J. Malaria in U.S. military forces: a description of deployment exposures from 2003 through 2005. Am J Trop Med Hyg. 2007;76(2):275–279.
- Armed Forces Health Surveillance Branch. Update: Malaria, U.S. Armed Forces, 2017. MSMR. 2018;25(2):2–7.
- Kotwal RS, Wenzel RB, Sterling RA, Porter WD, Jordan NN, Petruccelli BP. An outbreak of malaria in US Army Rangers returning from Afghanistan. JAMA. 2005;293(2):212–216. Erratum in: JAMA. 2005;293(6):678.
- Arakoda [package insert]. Washington, D.C.: 60º Pharmaceuticals; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210607lbl.pdf. Accessed on 29 Oct. 2018.