Abstract
The natural human sex ratio at birth (male:female) slightly favors males, and altered sex ratios might be indicative of exposure to reproductive hazards. In the U.S. Navy submarine community, there is a widespread belief that submariners are more likely to father females, but corroborating scientific evidence is limited. To assess this, Department of Defense Birth and Infant Health Research program data were used to identify 7,087 singleton infants whose fathers were considered submariners. Chi-square tests and unconditional logistic regression models were used to compare the offspring sex ratio of male submariners with 2 other active duty populations and the U.S. population. The offspring sex ratio of male submariners was 1.048, which did not substantially differ from the sex ratio of each comparison population. Furthermore, this study found no meaningful variation in offspring sex ratio by length of submarine or military service or by rating.
What Are the new findings?
Contrary to previous studies, this large, record-based analysis found no evidence to suggest the offspring sex ratio of male active duty U.S. Navy submariners is different from that of other active duty populations or the U.S. population as a whole.
What Is the Impact on Readiness and Force Health Protection?
This study's null findings suggest that submariners are not likely exposed to reproductive hazards in the workplace that alter offspring sex ratio. Current safety measures sufficiently protect the submariner force from such harmful exposures.
Background
Sex ratio is conventionally defined as the proportion of male to female live births in a given population. The natural human sex ratio at birth slightly favors males, with about 104 to 106 males born for every 100 females.1,2 Though considered a stable measure, sex ratio has steadily declined in most North American and European countries over the past several decades, albeit modestly.2–4 In the U.S., the sex ratio at birth decreased from 1.055 in 1940 to 1.048 in 2002;1 in 2016, the sex ratio of all live born U.S. infants was 1.047.5
Offspring sex ratio is often used in demographic, environmental, and occupational studies to assess the impact of certain exposures on reproductive and endocrine health.6–8 Because low sex ratios have been linked to reduced sperm quality and quantity,9–11 some postulate that a low offspring sex ratio is an early indicator for exposure to reproductive hazards or damage to the male reproductive system.
In the U.S. Navy submarine community, there is a widespread and longstanding belief that male submariners are more likely to father females than males; however, scientific evidence in support of this belief and biologic plausibility are limited. A 1970 record-based study from the Naval Submarine Medical Research Laboratory found a higher proportion of female offspring among male Navy personnel serving aboard nuclear-powered submarines than among the general U.S. population.12 A 2004 survey-based study did not corroborate this finding, but it did report a decrease in offspring sex ratio with additional time in the submarine community and detected lower sex ratios among submariners with certain naval ratings (i.e., occupational specialties), such as sonar technicians.13 A 2019 electronic survey-based study designed to assess whether male submariners have an altered offspring sex ratio found a low offspring sex ratio among respondents (sex ratio=0.95), particularly among those who reported being on sea duty (i.e., having a submarine-based job) at the time of conception (sex ratio=0.88), but no trends over time in the community were detected nor were there apparent differences by occupational speciality.14 However, as noted by the authors of the 2019 study, the fact that potential respondents were informed of the purpose of the survey likely introduced selection bias in favor of those who endorsed or held a belief that higher ratios of female offspring are associated with sea duty.14 While no other studies have investigated the offspring sex ratio of U.S. submariners, a cross-sectional survey of military men in the Royal Norwegian Navy found lower sex ratios among men with high degrees of exposure to radiofrequency electromagnetic fields, an occupational exposure also common among U.S. submariners.15
The present report used a record-based approach to assess whether male U.S. Navy submariners have an atypical offspring sex ratio, a possible indicator for exposure to reproductive hazards. In order to better elucidate the relationship between paternal submariner occupation and offspring sex ratio, this study examined whether sex ratio differed by length of submarine assignment or military service or by paternal occupational specialty.
Methods
This study utilized records from the Department of Defense (DOD) Birth and Infant Health Research (BIHR) program, an ongoing population-based surveillance effort established in 1998 to identify live births and associated outcomes among DOD beneficiaries.16 In brief, this effort gathers demographic, personnel, and occupational data from the Defense Manpower Data Center (DMDC) and electronic administrative medical data from the Military Health System Data Repository. The primary BIHR program population consists of all infants born to DOD beneficiaries from 1998–2015. Medical encounters through the infant's first year of life are coded with International Classification of Diseases, 9th/10th Revision, Clinical Modification (ICD-9-CM/ICD-10-CM) diagnostic codes, which are used to define the live birth population and health outcomes of interest. In this report, ICD-10 codes are used for encounters only in Oct. 2015 and later. Same-sex multiple infants are excluded from BIHR program data because of difficulty distinguishing their medical records. Estimated gestational age (EGA) is derived from ICD codes; date of last menstrual period (LMP) is calculated by subtracting EGA from delivery date; and date of conception is calculated by adding 2 weeks to date of LMP.
Infants were included in this study if their father was an active duty member of the U.S. Navy assigned a submarine-specific unit identification code (UIC) within 3 months before their conception; this timeframe was used to capture the period of spermatogenesis, which is estimated to last 74–120 days. Using DMDC personnel records, complete service histories—including information on assigned UICs—were obtained for all active duty sailors who began their service in 2000 or later. If an individual's assigned UIC was associated with a nuclear-powered, general-purpose attack submarine (SSN), ballistic missile submarine (SSBN), or cruise missile submarine (SSGN), they were considered a submariner and are referred to as such throughout this report. Infants resulting from multiple births were excluded from the analysis.
Of note, SSBNs and SSGNs are 2-crew submarines; in other words, sailors assigned to these submarines may be in an "on-crew" phase (when they would report to the submarine) or an "off-crew" phase (when they would report elsewhere); however, this study was unable to distinguish between on-crew and off-crew phases. Because the current analysis sought to assess the offspring sex ratio of fathers whose primary duties were aboard an underway submarine, sensitivity analyses excluding submariners assigned SSBN/SSGN-associated UICs were conducted; this subpopulation consisted of singleton infants born from 2001–2015 to male active duty submariners assigned SSN-specific UICs during preconception.
Three comparison populations were identified to assess whether the offspring sex ratio of active duty male submariners was atypical. Two comparison populations were derived from BIHR program data and included all singleton live births between 2001 and 2015 among 1) all male active duty U.S. Navy sailors and 2) all active duty military service men. The third comparison group was drawn from the U.S. population; information on the sex of all live births from 1995 through 2016 was obtained from the Centers for Disease Control and Prevention's Wide-ranging ONline Data for Epidemiologic Research (WONDER) database.5 Contingency tables and chi-square tests were used to compare the offspring sex ratio of male active duty submariners with the offspring sex ratio of each comparison population.
In order to assess the potential cumulative effect of submariner occupation, the current study also examined whether offspring sex ratio differed by length of submarine assignment or length of military service. These exposures were categorized based on the distribution in the population; sex ratios with 95% confidence intervals (CIs) were calculated according to quadratic formulas for binomial proportions.17 Length of submarine assignment was defined by the consecutive number of months (categorized in years) an infant's father was assigned a submarine-specific UIC before their month of conception (<1 year, >1 to <2 years, >2 to <3 years, or 3+ years); end of consecutive submarine assignment was defined as the first month a sailor was not assigned a submarine-specific UIC according to DMDC personnel records. Total length of military service was calculated by counting the number of months (categorized as years) from the father's first date of enrollment in the U.S. military to their offspring's month of conception (<5 years, >5 to <10 years, or 10+ years).
In order to examine whether offspring sex ratio varied by paternal occupation, naval ratings were used to categorize enlisted submariners by their occupational specialty; ratings were used as proxies for occupational exposures relevant to submariners. Offspring sex ratios and 95% CIs for binomial proportions were calculated for each rating and compared with the offspring sex ratio and 95% CIs of the overall submariner population. In 2012, the ratings system was altered to offer more specificity for certain ratings (e.g., the rating "machinist's mate" was expanded to consist of machinist's mate, nuclear power; machinist's mate, non-nuclear, submarine weapons; and machinist's mate, non-nuclear, submarine auxiliary). Because of small sample sizes, these expanded ratings were not included in the current analysis.
To account for potentially confounding factors, additional analyses were conducted on a population of exposed and unexposed infants identified from BIHR program data. Infants were considered exposed if their father was assigned a submarine-specific UIC during preconception, while infants were considered unexposed if their father was an active duty military service man in any other community during preconception or a service man previously assigned a submarine-specific UIC but not during preconception. In addition to a binary exposure variable (i.e., submariner=yes/no), a cumulative exposure measure was created based on the number of consecutive months an infant's father was assigned a submarine-specific UIC before their month of conception. Unconditional multivariable logistic regression models were used to estimate the odds of siring a female for fathers assigned submarine-specific UICs during preconception (both binary and cumulative exposure), with adjustment for paternal age (continuous), maternal age (continuous), and paternal race/ethnicity (American Indian/Alaska Native, Asian/Pacific Islander, non-Hispanic white, non-Hispanic black, Hispanic, other, and unknown), as variation in sex ratio by these demographic characteristics exists.1 Covariate information was obtained from BIHR program data. For analyses of cumulative exposure, the independent variable was rescaled by a factor of 6, so the effect estimate is interpreted as the odds of siring a female for every 6 additional consecutive months of assignment to a submarine-specific UIC. To assess potential exposure misclassification, a sensitivity analysis excluding unexposed service men previously assigned a submarine-specific UIC but not during preconception (n=3,972) was conducted. All statistical analyses were performed using SAS/STAT© software, version 9.4 (2014, SAS Institute, Cary, NC).
Results
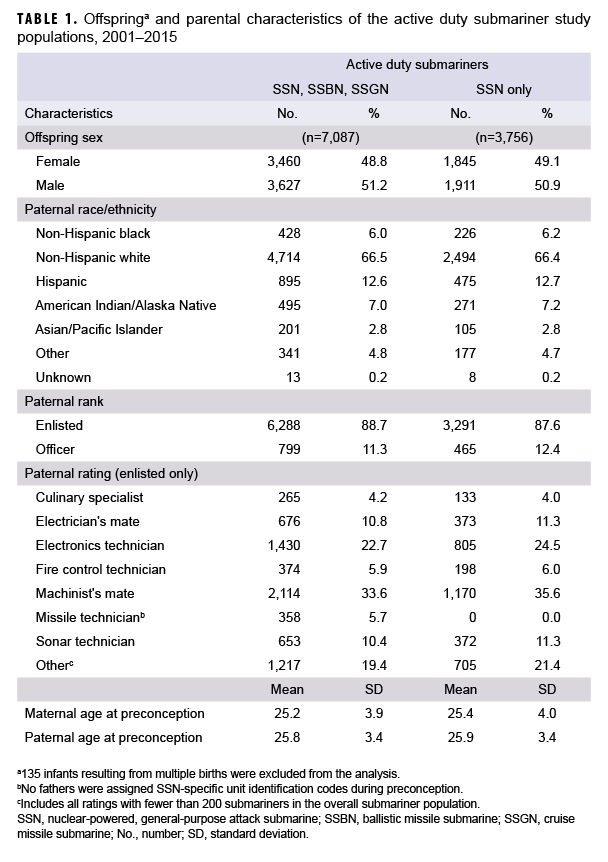
Demographic characteristics of offspring and parents, including information on paternal rank and rating, are outlined for both submariner study populations (Table 1). The current study identified a total of 7,087 singleton infants born to 5,931 male active duty submariners during 2001–2015. Excluded from this analysis were 135 infants resulting from multiple births. All submariner fathers were predominantly of non-Hispanic white or Hispanic race/ethnicity. Among enlisted submariners, the most common naval ratings were machinist's mate, electronics technician, electrician's mate, and sonar technician. Parental demographic and occupational characteristics were similar for both submariner populations; however, no fathers assigned SSN-specific UICs during preconception had a rating of missile technician, as only SSGN and SSBN submarines have ballistic missile systems.
In this population, offspring sex ratio differed by paternal race/ethnicity; the highest offspring sex ratios were observed among fathers who reported race/ethnicity as American Indian/Alaska Native (sex ratio=1.250), other (sex ratio=1.229), or Asian/Pacific Islander (sex ratio=1.185) (data not shown). Relatively lower offspring sex ratios were detected among Hispanic (sex ratio=1.096) and non-Hispanic white (sex ratio=1.019) fathers, while non-Hispanic black fathers were the only subgroup with an offspring sex ratio that favored females (sex ratio=0.911). Offspring sex ratios by race/ethnicity were similar when restricted to fathers assigned SSN-specific UICs during preconception (data not shown).
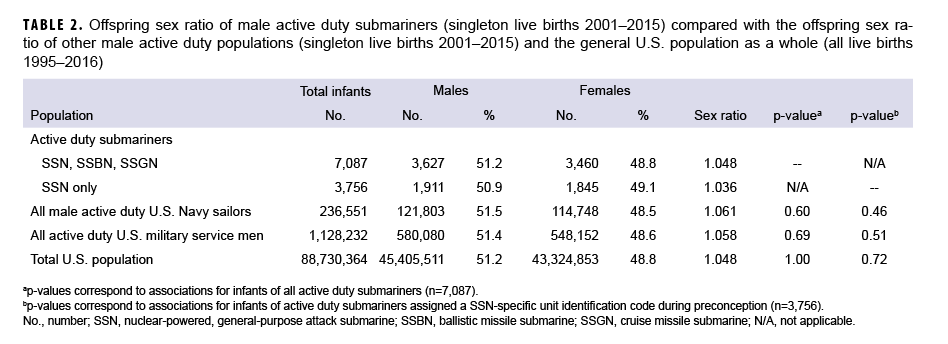
Among all singleton live births between 2001 and 2015, a total of 236,551 infants were identified among the comparison group of male active duty U.S. Navy sailors, and 1,128,232 infants were identified among the comparison group of active duty service men (Table 2). The third comparison group was derived from all live births in the general U.S. population from 1996 through 2016 and included 88,730,364 infants. The offspring sex ratio of male submariners did not differ substantially from the offspring sex ratio of male active duty U.S. Navy sailors, active duty U.S. military service men, or the U.S. population (Table 2).
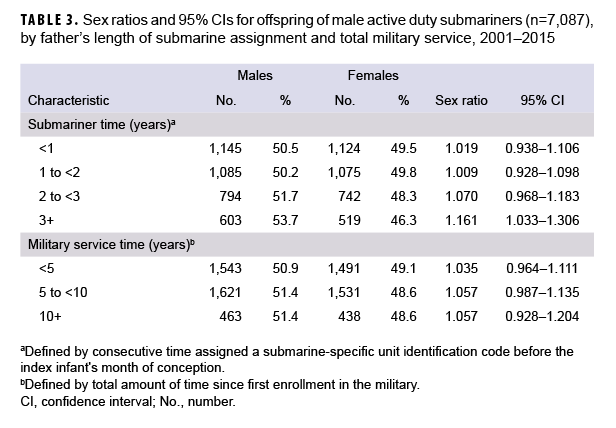
Considering cumulative exposure, submariners with less than 2 years of consecutive submarine assignment had lower offspring sex ratios than submariners with 2 or more years of consecutive submarine assignment (Table 3). There was little variation in offspring sex ratio by total length of military service. Offspring sex ratios were similar for the 3,756 singleton infants born to 3,220 fathers assigned SSN-specific UICs during preconception from 2001–2015 (data not shown).
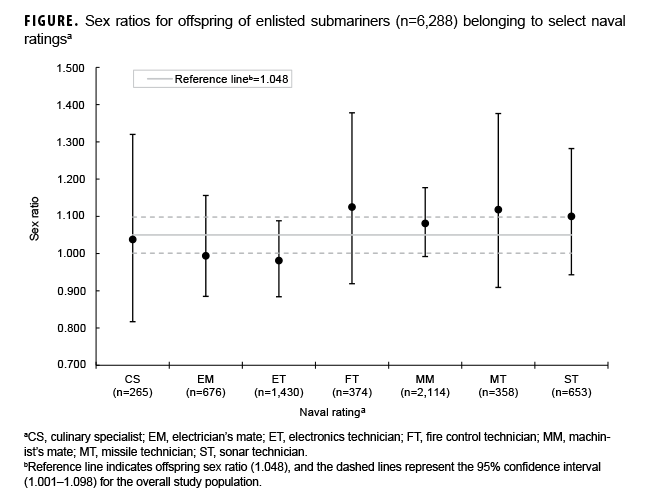
In analyses of occupational specialty, lower sex ratios were detected for enlisted fathers with a rating of culinary specialist (sex ratio=1.038), electrician's mate (sex ratio=0.994), and electronics technician (sex ratio=0.981), while higher sex ratios were observed for fathers with a rating of fire control technician (sex ratio=1.125), machinist's mate (sex ratio=1.081), missile technician (sex ratio=1.118), and sonar technician (sex ratio=1.100) (Figure). Overall, these estimates were imprecise and any observed differences by rating do not appear to be large or meaningful.
In supplementary analyses, the offspring sex ratio of male active duty submariners was compared with that of all active duty U.S. military service men, adjusting for parental age and paternal race/ethnicity. For infants whose fathers were active duty U.S. military service men, their parents were, on average, older at the time of preconception (mean ± standard deviation; maternal age=26.2±5.1; paternal age=27.4±5.4) (data not shown) than the parents of infants whose fathers were submariners (Table 1). Active duty U.S. military fathers were more likely to identify as non-Hispanic white (68.0%) or non-Hispanic black (12.2%) and less likely to identify as American Indian/Alaska Native (1.7%) (data not shown) than submariner fathers (Table 1). Multivariable logistic regression models estimated null associations between paternal submariner occupation and siring female offspring for both binary exposure (adjusted odds ratio [AOR]=1.01; 95% CI: 0.96–1.06) and cumulative exposure (AOR=1.00; 95% CI: 0.98–1.01). Results were similarly null for the population of infants whose fathers were assigned SSN-specific UICs during preconception, when all U.S. Navy sailors were used as the comparison group and when unexposed sailors previously assigned a submarine-specific UIC were excluded from analyses (data not shown).
Editorial Comment
The results of this large, record-based study suggest that the offspring sex ratio of male active duty U.S. submariners is normal. These findings conflict with results from previous studies of submariner offspring sex ratios, which detected lower sex ratios among all male submariners or by length of service and occupational specialty.12–14
While offspring sex ratio is known to differ by certain demographic characteristics (e.g., parental age and race/ethnicity),1 there are many suspected causes of variation in sex ratio. Perhaps the most well established is maternal stress, which is theorized to alter sex ratio through male-biased fetal losses. Studies have shown that mothers who experience catastrophic events in pregnancy, adverse periconceptional life events, or psychological stress during early gestation are more likely to experience fetal loss.18–20 Furthermore, evidence suggests these losses selectively cull frail males, thus resulting in a higher proportion of live born females among affected women.21,22 Other suspected causes of variation in sex ratio include parental hormone concentrations at the time of conception,23–25 ambient temperature during gestation,26 parental smoking status,27 and paternal occupation.28,29 Studies of the Chernobyl disaster suggest that exposure to high levels of environmental ionizing radiation increases the offspring sex ratio.30,31
The submarine environment is prone to a variety of potentially hazardous exposures, including radiation, disrupted circadian cycles, high stress, prolonged isolation, and altered oxygen and carbon monoxide levels. Of these, only radiation has been investigated in studies of paternal occupational exposure and offspring sex ratio, but evidence is conflicting.15,32–35 For submariners, the extent of exposure to radiation differs by occupational specialty. For example, all enlisted submariners with a rating of electrician& mate are nuclear-trained, but submariners with other ratings, such as machinist's mate and electronics technician, include those with and without nuclear training. However, it is important to note that sailors serving aboard submarines currently receive less total annual radiation exposure than they would if stationed ashore.36 Although the 2012 change to the ratings system better clarified which sailors worked with nuclear power, this study lacked the statistical power to conduct a sensitivity analysis for the years following this change. In this study, relatively low offspring sex ratios were detected for enlisted submariners with a rating of electrician's mate (sex ratio=0.994; 95% CI: 0.885–1.156) and electronics technician (sex ratio=0.981; 95% CI: 0.884–1.088), but a relatively high offspring sex ratio was detected for those with a rating of machinist's mate (sex ratio=1.081; 95% CI: 0.992–1.177). These findings are similar to those reported in a previous study of U.S. submariners.13 Although limited by imprecision, the current study did not find evidence to suggest that submariners' occupational specialty influenced offspring sex.
Because this was a record-based study, there was nondifferential misclassification of submariners and their exposure status, which would bias associations towards the null. Although historical personnel information was used to identify submariners based on their assigned UIC, it is possible that a sailor was assigned a UIC captured by DMDC records but did not actually serve aboard the corresponding vessel. To the authors' knowledge, there are no existing validation efforts that assess the accuracy of assigned UICs in military or Navy populations. Thus, it is unclear whether or how often UIC misclassification occurs. Furthermore, the assignment of a submarine-specific UIC does not necessarily indicate that sailors are serving aboard an underway submarine. While this study attempts to address this issue by conducting a sensitivity analysis of fathers assigned SSN-specific UICs during preconception, it cannot entirely account for all possible misclassification of submariner exposure status.
Additionally, because ICD codes were used to define EGA, date of conception (and therefore the preconception window used for exposure assessment) was also prone to nondifferential misclassification. However, a previous BIHR program validation study found ICD-9 codes provide an accurate assessment of EGA in this military population,37 thus limiting misclassification attributable to ICD coding errors. Additionally, the current study's record-based nature eliminates any recall or selection bias, which, given the widespread belief in the community that submariners are more likely to father females, has the potential to strongly affect a survey-based study of this population. The large sample of infants in the current study, prospectively collected over several years, sets it apart from most previous analyses of offspring sex ratio and submariners. Nonetheless, this study lacked the statistical power to detect small differences in offspring sex ratio.
The results of this study contradict the longstanding belief that male submariners are more likely to father females. These findings further indicate that submariners are not likely exposed to reproductive hazards in the workplace that alter offspring sex ratio and that current safety measures sufficiently protect the submariner force from such harmful exposures.
Author affiliations: Deployment Health Research Department in the Military Population Health Directorate, Naval Health Research Center, San Diego, CA (Dr. Hall, Ms. Bukowinski, Dr. Conlin); Leidos Inc., San Diego, CA (Dr. Hall, Ms. Bukowinski); Naval Aerospace Medical Institute, Pensacola, FL (LT Kramer); Innovative Employee Solutions, San Diego, CA (Dr. Conlin)
Conflicts of interest: None.
Disclaimer: The authors are military service members or employees or contract employees of the U.S. Government. This work was prepared as part of their official duties. Title 17, U.S.C. §105 provides that copyright protection under this title is not available for any work of the U.S. Government. Title 17, U.S.C. §101 defines a U.S. Government work as work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Report No. 19-09 was supported by the U.S. Navy Bureau of Medicine and Surgery under work unit no. 60504. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. The study protocol was approved by the Naval Health Research Center Institutional Review Board in compliance with all applicable Federal regulations governing the protection of human subjects. Research data were derived from an approved Naval Health Research Center, Institutional Review Board protocol number NHRC.1999.0003.
References
- Mathews TJ, Hamilton BE. Trend analysis of the sex ratio at birth in the United States. Natl Vital Stat Rep. 2005;53(20):1–17.
- Davis DL, Gottlieb MB, Stampnitzky JR. Reduced ratio of male to female births in several industrial countries: a sentinel health indicator? JAMA. 1998;279(13):1018–1023.
- Grech V, Vassallo-Agius P, Savona-Ventura C. Secular trends in sex ratios at birth in North America and Europe over the second half of the 20th century. J Epidemiol Community Health. 2003;57(8):612–615.
- Grech V. Sex ratios at birth in the British Isles over the past sixty years. Eur J Pediatr. 2013;172(4):525–528.
- CDC WONDER online database. Atlanta, GA: Centers for Disease Control and Prevention. https://wonder.cdc.gov/. Updated 18 Dec. 2018. Accessed 4 April 2019.
- Mocarelli P, Gerthoux PM, Ferrari E, et al. Paternal concentrations of dioxin and sex ratio of offspring. Lancet. 2000;355(9218):1858–1863.
- Mackenzie CA, Lockridge A, Keith M. Declining sex ratio in a first nation community. Environ Health Perspect. 2005;113(10):1295–1298.
- Hertz-Picciotto I, Jusko TA, Willman EJ, et al. A cohort study of in utero polychlorinated biphenyl (PCB) exposures in relation to secondary sex ratio. Environ Health. 2008;7:37.
- Arikawa M, Jwa SC, Kuwahara A, Irahara M, Saito H. Effect of semen quality on human sex ratio in in vitro fertilization and intracytoplasmic sperm injection: an analysis of 27,158 singleton infants born after fresh single-embryo transfer. Fertil Steril. 2016;105(4):897–904.
- Eisenberg ML, Murthy L, Hwang K, Lamb DJ, Lipshultz LI. Sperm counts and sperm sex ratio in male infertility patients. Asian J Androl. 2012;14(5):683–686.
- James WH. Male reproductive hazards and occupation. Lancet. 1996;347(9003):773.
- Bachmann WT. Births to Submarine Based Personnel—A Statistical Study. Groton, CT: Naval Submarine Medical Research Laboratory; 1970.
- Volk B. Evaluating the sex ratio in the offspring of U.S. Navy submariners. Mil Med. 2004;169(11):890–893.
- Kramer K, Raiciulescu S, Olsen C, Hickey K, Ottolini M. Altered sex ratios in offspring of U.S. submariners urban legend or fact—Do submariners have more daughters? Mil Med. 2019; Jan 24 [Epub ahead of print].
- Baste V, Riise T, Moen BE. Radiofrequency electromagnetic fields; male infertility and sex ratio of offspring. Eur J Epidemiol. 2008;23(5):369–377.
- Ryan MA, Pershyn-Kisor MA, Honner WK, Smith TC, Reed RJ, Gray GC. The Department of Defense Birth Defects Registry: overview of a new surveillance system. Teratology. 2001;64(suppl 1):S26–S29.
- Spiegel MR. Theory and Problems of Statistics. New York, NY: McGraw-Hill; 1961.
- Bruckner TA, Catalano R, Ahern J. Male fetal loss in the U.S. following the terrorist attacks of Sept. 11, 2001. BMC Public Health. 2010;10:273.
- Hansen D, Moller H, Olsen J. Severe periconceptional life events and the sex ratio in offspring: follow up study based on five national registers. BMJ. 1999;319(7209):548–549.
- 2007;22(11):3009–3012.
- Bruckner T, Catalano R. The sex ratio and age-specific male mortality: evidence for culling in utero. Am J Hum Biol. 2007;19(6):763–773.
- Catalano R, Bruckner T. Secondary sex ratios and male lifespan: damaged or culled cohorts. Proc Natl Acad Sci U S A. 2006;103(5):1639–1643.
- James WH, Grech V. Offspring sex ratio: coital rates and other potential causal mechanisms. Early Hum Dev. 2018;116:24–27.
- James WH, Grech V. A review of the established and suspected causes of variations in human sex ratio at birth. Early Hum Dev. 2017;109:50–56.
- James WH. Proximate causes of the variation of the human sex ratio at birth. Early Hum Dev. 2015;91(12):795–799.
- Catalano R, Bruckner T, Smith KR. Ambient temperature predicts sex ratios and male longevity. Proc Natl Acad Sci U S A. 2008;105(6):2244–2247.
- Fukuda M, Fukuda K, Shimizu T, Andersen CY, Byskov AG. Parental periconceptional smoking and male: female ratio of newborn infants. Lancet. 2002;359(9315):1407–1408.
- Magnuson A, Bodin L, Montgomery SM. Father's occupation and sex ratio of offspring. Scand J Public Health. 2007;35(5):454–459.
- Dickinson HO, Parker L. Sex ratio in relation to fathers' occupations. Occup Environ Med. 1997;54(12):868–872.
- Grech V. The Chernobyl accident, the male to female ratio at birth and birth rates. Acta Medica (Hradec Kralove). 2014;57(2):62–67.
- Scherb H, Kusmierz R, Voigt K. Increased sex ratio in Russia and Cuba after Chernobyl: a radiological hypothesis. Environ Health. 2013;12:63.
- Hama Y, Uematsu M, Sakurai Y, Kusano S. Sex ratio in the offspring of male radiologists. Acad Radiol. 2001;8(5):421–424.
- Dickinson HO, Parker L, Binks K, Wakeford R, Smith J. The sex ratio of children in relation to paternal preconceptional radiation dose: a study in Cumbria, northern England. J Epidemiol Community Health. 1996;50(6):645–652.
- Maconochie N, Roman E, Doyle P, Davies G, Smith PG, Beral V. Sex ratio of nuclear industry employees' children. Lancet. 2001;357(9268):1589–1591.
- Terrell ML, Hartnett KP, Marcus M. Can environmental or occupational hazards alter the sex ratio at birth? A systematic review. Emerg Health Threats J. 2011;4:7109.
- Mueller TJ, Weishar TM, Hallwoth JM, Bonamer DG; Naval Nuclear Propulsion Program, Department of the Navy. Report NT-18-2: Occupational radiation exposure from U.S. Naval nuclear plants and their support facilities. https://www.energy.gov/sites/prod/files/2018/07/f53/NT-18-2.pdf. Published May 2018. Accessed 4 April 2019.
- Barrett JP, Sevick CJ, Conlin AM, et al. Validating the use of ICD-9-CM codes to evaluate gestational age and birth weight. J Registry Manag. 2012;39(2):69–75.