Abstract
The Naval Infectious Diseases Diagnostic Laboratory (NIDDL) serves as a reference clinical laboratory that supports Department of Defense (DOD) military treatment facilities worldwide in the detection and identification of high-risk and emerging infectious diseases. Since the emergence of Zika virus (ZIKV) in the Western Hemisphere in 2016, the NIDDL has been a central hub for ZIKV testing for DOD personnel and beneficiaries. Samples collected during patients' clinical evaluations were screened for evidence of possible exposure to ZIKV using molecular and serological methods. An in-house ZIKV plaque reduction neutralization test was used to confirm the presence of ZIKV immunoglobulin M antibody. Of 1,420 individuals tested, ZIKV infection was confirmed by quantitative real-time polymerase chain reaction (PCR) assay in 11 (0.8%); an additional 26 recent flaviviral infections (possibly ZIKV) were identified based on serology (1.8%). These findings contribute to the understanding of the burden of ZIKV infections among DOD personnel and beneficiaries and highlight the role of the NIDDL in clinical diagnosis during emerging infectious disease outbreaks.
What Are the New Findings?
In 2016–2017, the NIDDL tested samples from 1,420 individuals and confirmed 11 cases of ZIKV infection by PCR assay and 26 flavivirus infections (possibly ZIKV) by serology. This report highlights the role of the NIDDL in clinical diagnosis during emerging infectious disease outbreaks and in understanding the burden of ZIKV infections within the DOD.
What Is the Impact on Readiness and Force Health Protection?
The NIDDL provides valuable information to characterize the burden of emerging diseases in U.S. military personnel and DOD beneficiaries, to inform risk assessments, and to guide diagnostics and countermeasure development. Given the low percentage of symptomatic cases among those infected, future serological studies should be undertaken to better determine the actual infection rates among U.S. military personnel.
Backgound
In 1947, Zika virus (ZIKV) was first discovered in the Zika Forest, Uganda, in the blood of a rhesus monkey.1 The following year, ZIKV was isolated from Aedes africanus mosquitoes.2,3 ZIKV is a member of the Flavivirus genus, which also includes dengue viruses, yellow fever virus, and West Nile virus. The first known human case of ZIKV infection was in 1953 in Nigeria.4 Although serosurveys found anti-ZIKV antibodies in people throughout Africa, India, and Southeast Asia,5 for more than 50 years after initial discovery, documented human cases of acute ZIKV infection were rare. Large numbers of human cases were not observed until 2007, when an outbreak occurred in Micronesia.6 Other outbreaks in the Pacific Ocean region followed, starting in 2013 in French Polynesia,7 with subsequent emergence in New Caledonia and American Samoa, among other locations.8 ZIKV was unknown in the Western Hemisphere until 2015, when ZIKV emerged in Brazil and rapidly spread across the country.
ZIKV is transmitted to humans most commonly through the bites of infected Aedes mosquitoes, but instances of sexual transmission have also been reported. ZIKV usually causes a mild disease with symptoms lasting about 1 week that may include low-grade fever, rash, arthralgia, arthritis, myalgia, headache, conjunctivitis, and edema. Severe cases involving hospitalization are uncommon and deaths are rare.9,10 In recent years, an increase in neurological complications has been associated with ZIKV infection.3,11,12 Following the 2015 Zika outbreak in Brazil, there was a dramatic increase in the number of babies with microcephaly born to mothers who had experienced ZIKV infections.3,11,12 On 1 Feb. 2016, the World Health Organization declared the ZIKV outbreak a "Public Health Emergency of International Concern."12 ZIKV became a growing concern to the U.S. military because of increased ZIKV transmission in the Western Hemisphere, an association between ZIKV infection and microcephaly and other birth defects, and a potential for sexual transmission.
In response, the Naval Infectious Diseases Diagnostic Laboratory (NIDDL),13 with the assistance of the Centers for Disease Control and Prevention (CDC),14 provided support to military treatment facilities (MTFs) in testing patients for ZIKV and other arboviruses (e.g., dengue virus [DENV] and chikungunya virus [CHIKV]) in returning military travelers and their beneficiaries. The NIDDL is certified by the Clinical Laboratory Improvement Amendments (CLIA) program, is accredited by the College of American Pathologists (CAP), and serves as a clinical reference laboratory specializing in the detection and identification of high-risk and emerging infectious diseases for clinicians at U.S. military health care facilities. This report summarizes laboratory testing at the NIDDL over the course of the ZIKV epidemic (29 Jan. 2016 through 31 Dec. 2017) and provides context for the NIDDL's role in diagnostic testing for the Department of Defense (DOD) during emerging disease outbreaks.
Methods
Population and inclusion criteria
An algorithm14 for recommended ZIKV testing was provided to health care practitioners at MTFs.13 The algorithm included criteria for testing of individuals with Zika-like illness and travel history to endemic regions, individuals with Zika-like illness and possible sexual exposure with or without travel history, asymptomatic pregnant women with possible ZIKV exposure, and neonates with or without microcephaly with potential ZIKV exposure. However, health care providers often sent samples from asymptomatic men and non-pregnant women who had traveled to ZIKV-affected areas and who were concerned about infection. Therefore, testing was performed on serum or urine collected from patients based on travel to ZIKV high-risk areas, sexual contact with a partner who had traveled to a ZIKV high-risk area (especially for pregnant women), or symptoms possibly indicative of ZIKV infection associated with travel. Seventy-four MTFs sent samples, most of which were from patients with either possible sexual or travel exposure. One hundred seventy-nine samples with either no reported exposure or with no data regarding exposure or symptoms were submitted to the NIDDL.
Specimens
Zika genomic material can be found in bodily fluids, such as semen, urine, breast milk, saliva, and amniotic fluid.15 ZIKV persists longer in urine (up to approximately 14 days) than it does in blood.16,17 Based on the CDC guidelines, the health care facilities were instructed to send both urine and serum for polymerase chain reaction (PCR) testing.14 All samples (serum and/or urine) were collected as part of routine clinical care when a patient visited an MTF to see a health care practitioner for acute care, routine screenings, or for other concerns. Samples were sent to the NIDDL for reference laboratory support during the ZIKV epidemic. Data for most samples were collected from manual logs submitted with the samples or from the Composite Health Care System, a database used by the Military Health System (MHS) to document patients' health information and history, electronically order laboratory and radiology tests/services, retrieve test results, and order and prescribe medications. The submitting MTFs were asked to provide the following information: patient demographics, date of exposure, geographic region of exposure or region of travel for sexual partner, symptoms and time of onset, pregnancy status, and trimester of exposure. However, not all data fields were complete with each submission. All data used in preparing this report were de-identified following release of clinical results to the ordering physician.
Laboratory testing
In the laboratory, total nucleic acid was extracted from samples using an automated system, the QIAGEN EZ1 Advanced XL with the EZ1 DSP Virus Kit (QIAGEN, Germantown, MD). Serum and urine collected from suspected ZIKV-infected patients were tested using the Trioplex quantitative real-time polymerase chain reaction (RT-PCR)16 assay for multiplex detection of ZIKV, DENV, and CHIKV on the Applied Biosystems (ABI) 7500 Fast Dx Real-Time PCR Instrument using the SuperScriptTM III PlatinumTM One-Step qRT-PCR Kit (ThermoFisher Scientific, Waltham, MA).
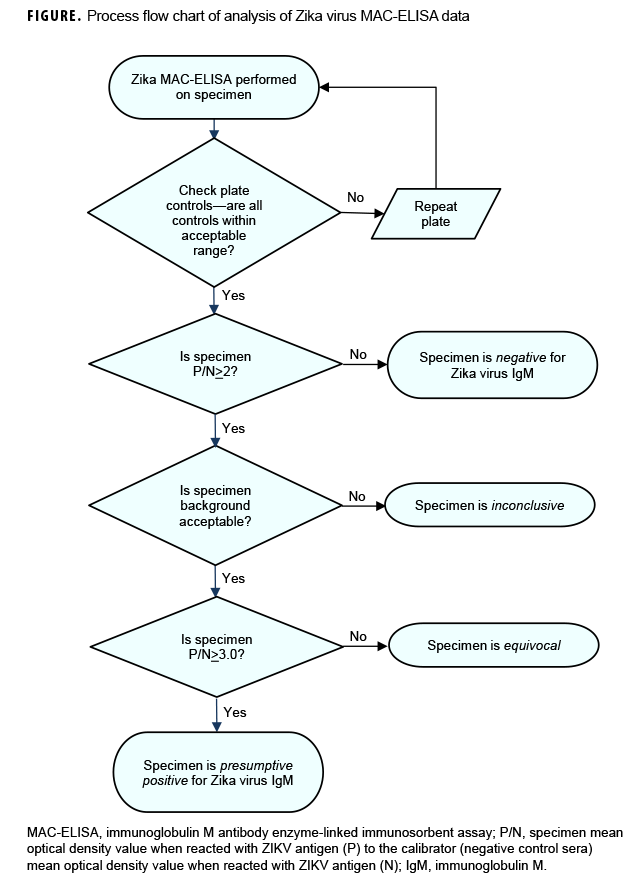
In line with established standards, all sera were tested using the ZIKV immunoglobulin M (IgM) antibody capture enzyme-linked immunosorbent assay (ZIKV MAC-ELISA). The primers and probes for Trioplex qRT-PCR16 and ZIKV MAC-ELISA antigens18 were provided by the CDC arbovirus laboratory through the Laboratory Response Network (LRN). Plate controls were interpreted and results were reported as presumptive positive, equivocal, negative, or inconclusive (i.e., results uninterpretable because of high background optical density [OD]) (Figure). The specimen P/N value, which is the ratio of the specimen mean OD value when reacted with ZIKV antigen (P) to the calibrator (negative control sera) mean OD value when reacted with ZIKV antigen (N), was calculated. A specimen P/N value <2 was interpreted as negative and no further testing or analysis was necessary. If the specimen P/N value was =2, then the background P/N value was assessed. The background P/N value is the ratio of the specimen mean OD value when reacted with ZIKV antigen (P) to the specimen mean OD value when reacted with Vero (negative) antigen (N). Both the specimen P/N value and the background P/N value must be =2 for the plate and specimens to be considered valid. The samples were deemed equivocal if the ratio was =2.0 P/N <3.0 or presumptive positive if the ratio was P/N =3.0. If the P/N value was =2 but the background value was <2, the specimen was deemed inconclusive. Inconclusive samples were re-tested and, if possible, another serum sample was requested from the submitting MTFs.
All inconclusive, equivocal, and presumptive positive specimens were referred to the in-house ZIKV plaque reduction neutralization test (PRNT).19 A low passage of ZIKV strain PRVABC59 was used to detect neutralizing antibody to ZIKV. The ZIKV PRNT titer is the reciprocal of the last serum dilution to show 90% plaque reduction. The cutoff dilution for positivity for PRNT was set at 1:10 as recommended in the CDC guidance for Zika testing.14 The PRNT is more specific than many other serological tests for the diagnosis of some viruses.20 To ensure quality of all results, CDC provided and evaluated the proficiency testing as well as the protocols for all 3 assays.
Although the CDC laboratory criteria for diagnosis of ZIKV infection21 includes ruling out DENV infection by PRNT, DENV PRNT was not established in the NIDDL and thus was not performed. We adopted a modified version of the CDC laboratory definition. Confirmed "recent ZIKV infection" was defined based on detection of viral ribonucleic acid (using the Trioplex qRT-PCR). "Recent Flavivirus infection, possible ZIKV" was defined as positive ZIKV IgM with positive ZIKV neutralizing antibody titers. In some cases, samples were sent to the CDC for DENV PRNT testing when requested by the clinician for clarification of results and patient management.
Analysis
Data were manually entered into a Microsoft Access database (2013, Microsoft Corporation, Redmond, WA). Data cleaning and extraction were performed using Microsoft Excel 2013 (2013, Microsoft Corporation, Redmond, WA) and R, version 3.2.1 (2015, R Foundation for Statistical Computing, Vienna, Austria). Data were analyzed using a combination of R, version 3.2.1, and Stata, version 13.0 (StataCorp LP, College Station, TX).
Results
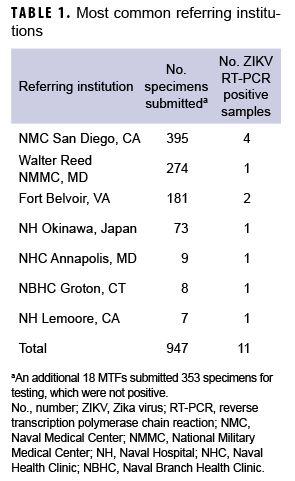
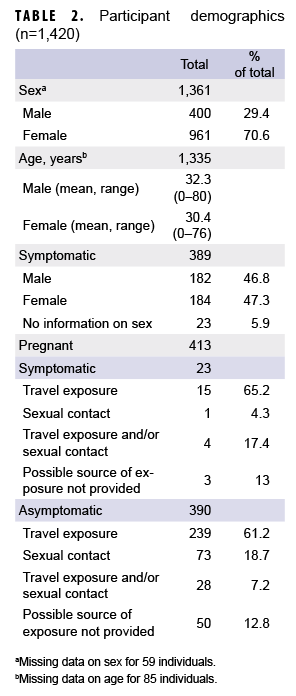
Between 29 Jan. 2016 and 31 Dec. 2017, samples from 1,420 individuals were received from DOD medical facilities around the world, including the U.S., Japan, Germany, Cuba, Guam, Spain, and Italy (Table 1). Four hundred and twelve of these individuals had clinical symptoms consistent with ZIKV infection, 852 were asymptomatic but with epidemiologic linkage to ZIKV cases, and 179 had either no reported exposure to ZIKV or no data regarding exposure or symptoms. There were 1,361 samples that included information on sex, 1,330 that included information on age, and 1,241 that included information on whether the patient was symptomatic or asymptomatic. In light of concerns for impact on fetal development, the majority of samples tested were from females (961/1,361; 70.6%; Table 2). The median age was 30 years (0–80 years); the majority (1,161/1,330; 87.3%) were between the ages of 18 and 40 years (data not shown).
Of the samples that included information on symptom status of the individual, (n=1,241; 87.8%), 389 (31.3%) were categorized as having come from symptomatic patients and 852 (68.7%) were categorized as having come from asymptomatic patients (data not shown). Of the samples identified as having come from symptomatic patients and that included information on travel to endemic regions (n=329), nearly all were associated with recent travel to endemic regions (n=314/329; 95.4%) or travel as well as potential sexual exposure (n=11/329; 3.3%). However, 2 (0.6%) of the samples were from newborns with reported ZIKV-infected mothers and 2 (0.6%) were from females without recent travel but with sexual contact with partners recently returning from endemic regions (data not shown). Of the samples from asymptomatic individuals that included information on travel (n=784), 615 (78.4%) were associated with recent travel to endemic regions, 120 (15.3%) were associated with sexual contact with a partner with recent travel history, 45 (5.7%) were associated with potential travel and sexual exposure, and 4 (0.5%) were associated with potential congenital exposure (data not shown).
Samples were received from 413 pregnant women; 23 (5.6%) of the women were symptomatic and 390 (94.4%) were asymptomatic (Table 2). Of the patients who were asymptomatic and pregnant, testing was indicated because of geographical exposure such as travel to or living in endemic regions (n=239; 70.3%), sexual contact with potential ZIKV-infected partners (n=73; 21.5%), or both (n=28; 8.2%).
Overall, individuals with reported potential travel exposure (n=985), including travel and sexual exposure, reported having visited or lived in Central America or the Caribbean (n=466; 47.3%), the Asia-Pacific region (n=246; 25.0%), South America (n=59; 6.0%), Africa (n=44; 4.5%), and/or potentially endemic regions of the U.S. (n=132; 13.4%); 57 individuals had no travel location provided (data not shown).
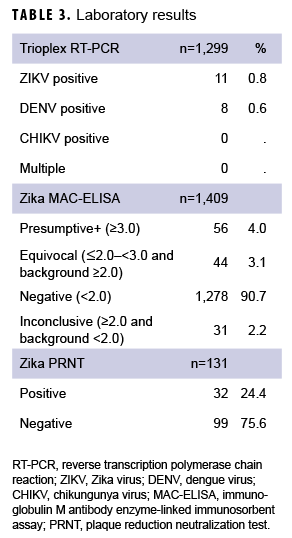
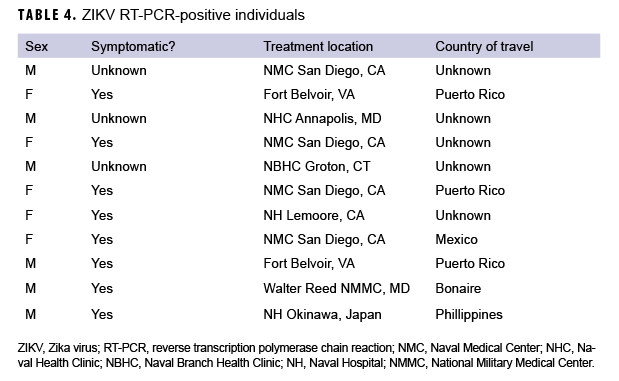
A variety of samples (n=1,948) from the 1,420 patients were submitted for testing, including, but not limited to, serum (n=891); serum and urine (n=500); serum and whole blood (n=15); serum, whole blood, and urine (n=5); urine only (n=4); semen and urine (n=1); semen, urine, and sputum (n=1); and semen only (n=1) (data not shown). For some patients, multiple samples were received, and for others, just 1 sample was received. Serum samples were tested using Zika MAC-ELISA, PRNT, and the CDC Trioplex qRT-PCR assay, whereas urine and semen samples were tested with the CDC Trioplex qRT-PCR assay only. Out of the 1,299 individuals tested using Trioplex qRT-PCR, there were 11 ZIKV-positive individuals (0.8%) (4 positive in serum, 6 in urine, and 1 in both sample types) and 8 (0.6%) DENV-positive individuals (3 positive in serum and 5 positive in both serum and urine) (Table 3). None of the samples tested were positive for CHIKV (Table 3). Among the 11 samples that were qRT-PCR-positive for ZIKV, 8 came from symptomatic patients, of whom 5 had travel histories to endemic locations in the Western Hemisphere (i.e., Puerto Rico, Mexico, or Bonaire) and 1 had been to the Philippines (Table 4). Travel histories were not available for the other 2 symptomatic individuals with ZIKV-positive samples (Table 4). The remaining 3 ZIKV-positive samples lacked information on symptom status and travel history.
Serum samples from 1,409 individuals were screened by Zika MAC-ELISA (Table 3). Of these, 56 were classified as presumptive positive, 44 were equivocal, 1,278 were negative, and 31 were inconclusive. All positive, equivocal, or inconclusive samples were analyzed by Zika PRNT for confirmation. Among Zika IgM-positive samples, 52% (29/56) were PRNT positive; among equivocal or inconclusive only samples, 4% (3/75) were PRNT-positive. Excluding the serology results from qRT-PCR-confirmed ZIKV and DENV infections, serological testing resulted in 26 individuals classified as "Recent Flavivirus infection, possible ZIKV." Eleven of these individuals were symptomatic, 11 were asymptomatic, and 4 had no history provided. Therefore, combining molecular and serological testing, a total of 37 individuals had laboratory results consistent with recent ZIKV infection: 11 confirmed and 26 possible recent ZIKV infections (Table 3).
Of the 413 pregnant women screened, 8 (1.9%) were considered to have recent flavivirus, possible ZIKV infections by Zika MAC-ELISA with PRNT confirmation. One was symptomatic. All 8 ZIKV-positive pregnant women reported recent travel to ZIKV-endemic areas, including Puerto Rico, the Philippines, and Brazil, and therefore mosquito transmission during travel rather than sexual contact was considered the likely route of exposure (data not shown).
Additionally, 9 infants were tested for recent ZIKV infection, including 3 with reported microcephaly. None of the 3 infants with reported microcephaly tested positive for ZIKV by any of the tests. One infant without reported microcephaly tested inconclusive by Zika MAC-ELISA but was positive by PRNT, most likely due to circulating maternal antibody to ZIKV; the infant's mother reported recent travel to Puerto Rico (data not shown) and also tested positive by Trioplex qRT-PCR, MAC-ELISA, and PRNT.
Editorial Comment
Starting in Jan. of 2016, the NIDDL provided support to MTFs by testing for ZIKV and other arboviruses (DENV and CHIKV) in returning military travelers and their beneficiaries. Following the realization that ZIKV can be transmitted sexually, the NIDDL began screening females of childbearing age and those reporting pregnancy after sexual contact with a member "exposed" or symptomatic following travel to a region with ZIKV risk.
The NIDDL is a CLIA-certified and CAP-accredited infectious disease laboratory that serves multiple roles within the DOD, from clinical diagnostic laboratory to reference laboratory for the detection and identification of high-risk emerging infectious diseases even during large outbreaks. In addition, the NIDDL maintains a repository of samples for assay development and quality assurance. The NIDDL partners with CDC and the LRN to support surveillance studies of emerging infections among DOD personnel.
ZIKV poses a challenge for laboratory confirmation. Infection is often asymptomatic, and even symptomatic cases are characterized by low-grade undifferentiated febrile illness that is difficult to distinguish from illness caused by other co-circulating pathogens, such as DENV, CHIKV, rickettsial pathogens, and malaria. Serological confirmation is particularly challenging because of cross-reaction with other flavivirus antibodies, particularly DENV. The NIDDL provides diagnostic confirmation, not only using molecular methods such as qRT-PCR, but also ELISA with follow-up diagnostic PRNT confirmation.
The NIDDL experienced several challenges during the current study. First, testing guidelines were disseminated to health care practitioners, but they were not always followed. For example, many samples were submitted from asymptomatic men and asymptomatic non-pregnant women without any travel history or other risk factors, including sexual contact with a symptomatic partner who had traveled to a high-risk location. In addition, there were occasional instances of broken containers or samples that leaked during shipment as well as the submission of incorrect orders. Finally, patient travel/sexual history was missing for different subsets of samples. Despite these challenges, the NIDDL successfully tested all of the received samples, reported the results to the requesting facility, and reported results to CDC through the LRN messenger system.
The current study identified 37 confirmed and possible ZIKV infections (2.6%), including 19 symptomatic infections. The ZIKV infections reported here represent a subset of all cases reported across the MHS. Poss and colleagues22 reported 156 confirmed cases between Jan. and Nov. 2016, including 5 in pregnant beneficiaries. Those reported cases were nearly all symptomatic, whereas the current study detected a number of asymptomatic infections among individuals with travel exposure to endemic regions. Given that the majority of ZIKV infections are asymptomatic or at least subclinical,23 incidence rates among military personnel will be largely undercounted, as predominantly symptomatic cases will be reported. Additionally, 8 pregnant women tested positive for ZIKV (1.9% of all pregnant women tested), all by Zika MAC-ELISA and PRNT serology. Exposure through travel, rather than sexual exposure, was likely responsible for all of these infections.
DOD personnel represent a unique population in many ways since they are deployed globally, often in austere environments, and thus are exposed to locally circulating pathogens. The NIDDL provides valuable information to characterize the burden of emerging diseases in U.S. military personnel and DOD beneficiaries, inform risk assessments, and guide diagnostics and countermeasure development. Because of the sequelae of Zika, an urgent need exists for a vaccine. There are a variety of ZIKV vaccines currently in development that employ a range of technologies and approaches (e.g., inactivated ZIKV, protein nanoparticle, synthetic peptide, DNAbased, virus-like particle, thermostable mRNA-based, live modified vaccinia virus Ankara, recombinant protein vaccines, and self-amplifying mRNA platform).15 However, the vaccine must elicit protective immunity regardless of mode of transmission, be free of neurological side effects, and protect healthy adults, young children, pregnant women, and unborn fetuses.15 Assessment of clinical efficacy when the majority of ZIKV infections are asymptomatic poses an additional challenge.15
Future work will involve sequencing of ZIKV isolates and looking for linkages between them, as the women were infected in a variety of locations, including the Caribbean, South America, and Asia. The NIDDL will also develop and validate a DENV PRNT assay to assess cross-reactivity and verify the presence of ZIKV antibodies. Additional work is needed to determine if pathogenesis of the organism varies in relation to sexual transmission when compared to vector-borne transmission. Control of mosquito populations is imperative to prevent further spreading of the organism. Moreover, in light of the low percentage of symptomatic cases among those infected, future cross-sectional serological studies should be undertaken to better determine the actual infection rates among U.S. military personnel.
Author affiliations: Naval Infectious Diseases Diagnostic Laboratory, Viral and Rickettsial Diseases Department, Infectious Diseases Directorate, U.S. Naval Medical Research Center, Silver Spring, MD (Mr. Sugiharto, Ms. Widjaja, CDR Williams, and LCDR Simons); U.S. Food and Drug Administration (CDR Myers); Armed Forces Health Surveillance Branch, Silver Spring, MD (Ms. Hartman); Cherokee Nation Technologies, Silver Spring, MD (Ms. Hartman)
Acknowledgments: The authors thank Dr. Brett Forshey (Armed Forces Health Surveillance Branch) for reviewing and editing the manuscript and for his database analysis contribution. Funding for this study was provided by the Armed Forces Health Surveillance Branch and its Global Emerging Infections Surveillance and Response section.
References
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–520.
- Song BH, Yun SI, Woolley M, Lee YM. Zika virus: history, epidemiology, transmission, and clinical presentation. J Neuroimmunol. 2017;308:50–64.
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. New Engl J Med. 2016;374(20):1981–1987.
- Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48(2):139–145.
- Lessler J, Chaisson LH, Kucirka LM, et al. Assessing the global threat from Zika virus. Science. 2016;353(6300):aaf8160.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. New Engl J Med. 2009;360(24):2536–2543.
- Cao-Lormeau VM, Roche C, Teissier A, et al. Zika virus, French Polynesia, South Pacific, 2013. Emerg Infect Dis. 2014;20(6):1085–1086.
- Petersen LR, Jamieson DJ, Honein MA. Zika Virus. New Engl J Med. 2016;375(3):294–295.
- 9Paixao ES, Barreto F, Teixeira Mda G, Costa Mda C, Rodrigues LC. History, epidemiology, and clinical manifestations of Zika: a systematic review. Am J Public Health. 2016;106(4):606–612.
- Chang C, Ortiz K, Ansari A, Gershwin ME. The Zika outbreak of the 21st century. J Autoimmun. 2016;68:1–13.
- Wikan N, Smith DR. Zika virus: history of a newly emerging arbovirus. Lancet Infect Dis. 2016;16(7):e119–e126.
- World Health Organization. WHO Director-General summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome. 1 Feb. 1 2016. https://www.who.int/news-room/detail/01-02-2016-whodirector-general-summarizes-the-outcome-of-theemergency-committee-regarding-clusters-of-microcephaly-and-guillain-barr%C3%A9-syndrome. Accessed 31 Jan. 2018.
- Naval Infectious Disease Diagnostic Laboratory. Interim Zika Virus Testing Criteria. http://www.med.navy.mil/sites/nmrc/SiteAssets/Interim%20Zika%20Virus%20Testing%20Criteria.pdf. Accessed 9 April 2018.
- Centers for Disease Control and Prevention. Updated Guidance for US Laboratories Testing for Zika Virus Infection. https://www.cdc.gov/zika/laboratories/lab-guidance.html. Accessed 13 May 2019.
- Poland GA, Kennedy RB, Ovsyannikova IG, Palacios R, Ho PL, Kalil J. Development of vaccines against Zika virus. Lancet Infect Dis. 2018;18(7):e211–e219.
- Centers for Disease Control. Trioplex Realtime RT-PCR Assay. 6 April 2017. https://www.cdc.gov/zika/pdfs/trioplex-real-time-rt-pcr-assay-instructions-for-use.pdf. Accessed 31 Jan. 2018.
- Gourinat AC, O'Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21(1):84–86.
- Centers for Disease Control and Prevention. Zika MAC-ELISA. 3 May 2017. https://www.cdc.gov/zika/pdfs/zika-mac-elisa-instructions-for-use.pdf. Accessed 31 Jan. 2018.
- Rabe IB, Staples JE, Villanueva J, et al. Interim guidance for interpretation of Zika virus antibody test results. MMWR Morb Mortal Wkly Rep. 2016;65(21):543–546.
- Shan C, Xie X, Ren P, et al. A rapid Zika diagnostic assay to measure neutralizing antibodies in patients. EBioMedicine. 2017;17:157–162.
- Centers for Disease Control and Prevention. Zika Virus Disease and Zika Virus Infection, 2016 Case Definition, Approved June 2016. https://wwwn.cdc.gov/nndss/conditions/zika/case-definition/2016/06/. Accessed 9 April 2018.
- Poss DE, Writer JV, Harris S. Zika virus infections in Military Health System beneficiaries since the introduction of the virus in the Western Hemisphere, 1 Jan. 2016 through 30 Nov. 2016. MSMR. 2016;23(12):7–11.
- Shuaib W, Stanazai H, Abazid AG, Mattar AA. Re-emergence of Zika virus: a review on pathogenesis, clinical manifestations, diagnosis, treatment, and prevention. Am J Med. 2016;129(8): 879.