DOD COVID-19 vaccine roll-out continues, eye on long-term readiness
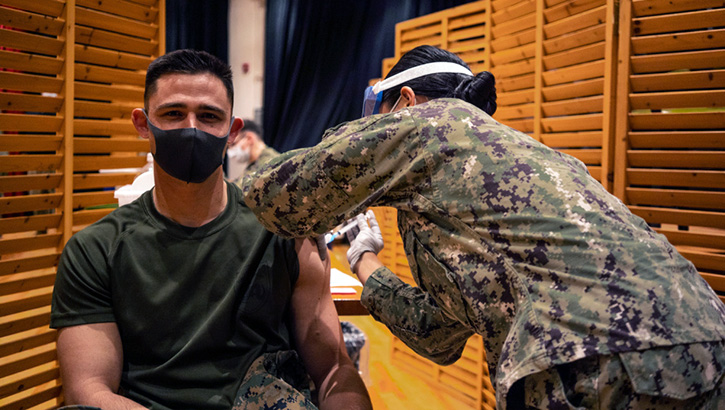 Marines with the 3rd Marine Expeditionary Brigade receive the Moderna COVID-19 vaccination shot on Camp Hansen, Okinawa, Japan on Jan. 20. Receiving the vaccine mitigates risks to military operations, allowing Marines to maintain their readiness and be able to respond to any crisis or contingency in the Indo-Pacific region. (Photo by Marine Cpl. Sarah Marshall, 3rd Marine Expeditionary Brigade.)
Marines with the 3rd Marine Expeditionary Brigade receive the Moderna COVID-19 vaccination shot on Camp Hansen, Okinawa, Japan on Jan. 20. Receiving the vaccine mitigates risks to military operations, allowing Marines to maintain their readiness and be able to respond to any crisis or contingency in the Indo-Pacific region. (Photo by Marine Cpl. Sarah Marshall, 3rd Marine Expeditionary Brigade.)
Moving hundreds of thousands of COVID-19 vaccines to more than 300 military bases at home and abroad is a monumental task, especially when the supply of the vaccines is still in the early stage. Perhaps more significant is the psychological impact of waiting to get a shot, understanding the facts about its safety, and wading through the daily barrage of media reports and a formidable rumor mill amid the deadliest virus to hit the United States in more than a century.
While the news has celebrated the current vaccines for being 95% or more effective to prevent illness from the virus, they remain among of the most important prevention methods to combatting the spread of COVID-19.
At a Pentagon press briefing Jan. 28, Joint Staff Surgeon Air Force Brig. Gen. Paul Friedrichs described the vaccine as a third layer of prevention against the disease, emphasizing that the shot, combined with public health measures and testing, will help control the pandemic.
He said the basic personal protection measures of physical distancing and wearing face coverings and masks are “not going to go away in the near term as we roll out the vaccines.”
That’s because research has not yet provided answers to key questions about how widely the vaccine can prevent the spread of the virus. “That’s difficult, [to know] the immediate assessment on the impact on readiness and training and operations,” said Air Force Col. (Dr.) Jessica Cowden, chief of infectious disease programs with the Defense Institute for Medical Operations, Joint Base San Antonio-Lackland in Texas.
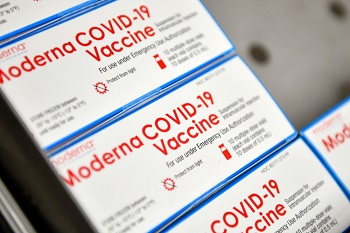 Boxes of Moderna COVID-19 vaccines are placed into a freezer at Aviano Air Base, Italy, Jan. 7, 2021. Each box holds 10 vials, with each vial containing approximately 10 doses of the vaccine. (Photo by Air Force Staff Sgt. K. Tucker Owen.)
Boxes of Moderna COVID-19 vaccines are placed into a freezer at Aviano Air Base, Italy, Jan. 7, 2021. Each box holds 10 vials, with each vial containing approximately 10 doses of the vaccine. (Photo by Air Force Staff Sgt. K. Tucker Owen.)
“The phase 3 vaccine trial is designed to evaluate efficacy — how well the vaccines work to prevent clinical infection, systematic COVID. They are not designed to evaluate the vaccine’s ability to prevent an asymptomatic infection, or transmission. So those questions are still there, and still unanswered.”
Cowden, who has been on assignment with the Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND) and is coordinating participation in the Phase 3 COVID-19 vaccine studies, was referring to one of many challenging aspects of COVID-19 — the way in which it can easily spread from infected people who show no symptoms. It’s why even with the high efficacy of the shots, mask-wearing, physical distancing, and quarantining must continue for the foreseeable future, she stressed.
Like any medication or vaccination, it's normal to wonder about the safety of what's going into your body, and defense health experts express high levels of confidence in the safety of the COVID-19 vaccine.
“On one level, it’s amazingly fast,” said Dr. Clay Holloway, director of the Office of Strategy and Integration at JPEO CBRND, where part of the office’s charge is “medical countermeasures” for the military and special operations. “On another level, it’s unfair to say that this is all the time that’s been put into it, because we’ve spent quite a while developing the messenger RNA platform, and understanding what it can do, what it can’t do. But there are limits to how much you can profile the safety. You’ve really got to get whatever it is you’re vaccinating against, and see how that interacts with the body and whether it’s effective or not.”
In other words, the mRNA vaccine has been around for a few years now, but the COVID-19 trials are the first real test of the impact of it. The testing was unprecedented, experts agree.
“It did take the whole nationwide effort, the whole of government, the whole of industry effort to bring these forward this fast,” Holloway said, adding that “the Food and Drug Administration does its job incredibly well. When you take a drug from them ... you can be pretty confident it’s going to work.”
He added how the COVID-19 trials were accelerated by doing a lot of things in parallel instead of in a series of steps, much different from the normal method. It means looking at the same number of people tested and generating the same amount of data.
“But again, that’s not normal, because we don’t normally have the entire nation’s resources all focused on one problem,” he said. “This was unique.”
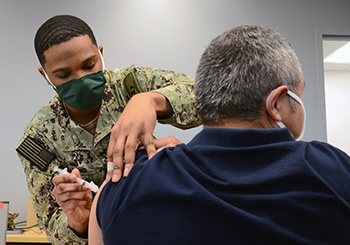 Navy Hospitalman Jaylen King, assigned to Navy Medicine Readiness and Training Command Corpus Christi, administers the Moderna COVID-19 vaccine to a patient at U.S. Coast Guard Sector Air Station Corpus Christi, Texas. NMRTC-CC is following the DOD’s COVID-19 vaccine distribution plan to implement a phased strategy to administer the vaccine to protect our fighting force and maintain readiness. (Photo by Dale Davis, Naval Health Clinic Corpus Christi, Texas.)
Navy Hospitalman Jaylen King, assigned to Navy Medicine Readiness and Training Command Corpus Christi, administers the Moderna COVID-19 vaccine to a patient at U.S. Coast Guard Sector Air Station Corpus Christi, Texas. NMRTC-CC is following the DOD’s COVID-19 vaccine distribution plan to implement a phased strategy to administer the vaccine to protect our fighting force and maintain readiness. (Photo by Dale Davis, Naval Health Clinic Corpus Christi, Texas.)
There are many other unknowns. How long can the vaccines live in the freezer? What about efficacy against new variants of the virus? When does the nation get to the point where enough people become immune to prevent the spread? Will you have immunity for life? Answers remains elusive, but waiting on every implication of a new vaccine is not what “emergency use authorization” is all about.
“We do know that it takes that second shot, or single booster [three or four weeks after the first], to get really good initial efficacy,” Cowden said. “We’ll have to see how long you keep that immunity, and that’s fairly common for different vaccines.”
With most vaccines, the idea is that you don’t get perfect protection forever, but good enough protection to keep you from becoming ill for a period, the doctors said. Take the new Shingrix vaccine, approved in 2017 to treat shingles, a painful, blistering illness. The Centers for Disease Control and Prevention recommends that healthy adults 50 and older get two doses separated by two to six months. In studies, Shingrix was more than 97% effective, and as of late 2019, protection stayed above 85% effectiveness at four years after vaccinations. Testing will continue, as with all vaccines.
Likewise, the COVID-19 vaccine needs to be tracked over time, Holloway said, to see how long the vaccinated “keep their antibodies up.” It could be that the virus needs an annual inoculation, like for seasonal influenza, but it is too early to tell. Those getting the vaccine now will not have to go back for testing, but should simply stay up to date on vaccine news to know whether they need to be re-vaccinated.
As far as the physical impact of the shots, or side effects, some people have none. Some get a sore arm. Some get headaches, or a fever. A very small number get a bad allergic reaction, but it is one that is usually seen within 15 or 20 minutes. Just like with giving blood, you are asked to stick around for a while after getting the relatively painless jab.
Despite the many articles and constant stream of information about the safety of the COVID-19 vaccines and the means of slowing the virus, the anti-vaccine sentiment remains stubborn, and is impacting efforts at effective messaging.
Recently, for example, there were many negative comments on the TRICARE Facebook page when facts on the vaccine were presented. Beneficiaries shared that they were unwilling to get a COVID-19 vaccine without knowing the long-term side effects, or that they think the vaccine is a bigger risk than COVID-19 itself (you cannot acquire COVID from the vaccines). Experts caution to be wary about unproven theories via the so-called “rumor mill,” and seek credible information.
“COVID-19 vaccines are brand new products, in a pandemic-weary world that is flooded with social media information,” said Dr. Margaret Ryan, medical director of the Defense Health Agency’s Immunization Healthcare Division. “This environment may engender much misinformation. People should be encouraged to seek reliable information from universities, the Centers for Disease Control, the FDA, and DOD — especially the DHA Immunization Healthcare Division website.”
Ryan said that though it is not mandatory for military members, she encourages everyone to be vaccinated as soon as it is offered to them.
“People should feel confident in these highly effective vaccines that have reassuring safety profiles after millions of doses have been administered,” she said. “Receiving COVID-19 vaccination reduces one’s personal risk of infection, protects other people, and helps the world to eventually emerge from the pandemic.”