COVID-19 hinders blood donations during National Blood Donor Month
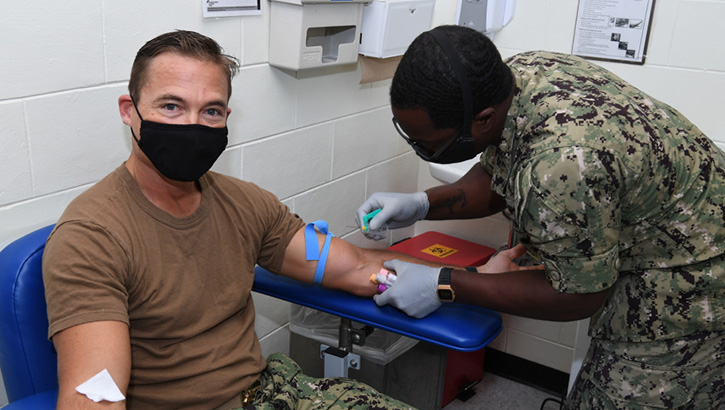 Navy Capt. R. Wade Blizzard, commanding officer of U.S. Navy Support Facility Diego Garcia, donates blood for the Navy Medicine Readiness and Training Units Diego Garcia walking blood bank on Dec. 17, 2020. The walking blood bank is a list of eligible donors who can provide blood in case of emergency. (U.S. Navy photo by Navy Seaman Apprentice Stevin Atkins)
Navy Capt. R. Wade Blizzard, commanding officer of U.S. Navy Support Facility Diego Garcia, donates blood for the Navy Medicine Readiness and Training Units Diego Garcia walking blood bank on Dec. 17, 2020. The walking blood bank is a list of eligible donors who can provide blood in case of emergency. (U.S. Navy photo by Navy Seaman Apprentice Stevin Atkins)
ASBP is the military's only blood donor program for service members, their families, retirees, veterans, and local communities worldwide, helping those in critical need. Both whole blood and COVID-19 Convalescent Plasma (CCP) from those who have recovered from COVID-19 remain in high demand. All 21 donor centers adhere to CDC guidelines to reduce the spread of COVID-19.
Getting people to donate a pint of their potentially life-saving blood has never been easy. And, like almost every aspect of modern life, COVID-19 has made it even harder.
With strong recommendations from the Centers for Disease Control to keep one’s distance from others and always wear a mask in public, volunteering to go to a facility for about 40 minutes to have blood drawn has not been a priority for many. And then there were the blood drive cancellations — too many to count, for months at a time.
“The public health guidelines to reduce interaction with others, social distancing, reducing time outside the home … it translates into a decreased donor turnout,” said Army Col. Jason Corley, director of the Army Blood Program, from the U.S. Army Medical Command at Joint Base San Antonio-Fort Sam Houston, Texas. “We’re no different from our civilian blood agency counterparts. They’ve been experiencing the same things since March. Everybody’s trying to do the right thing. Commanders and donors want to be safe and healthy. It makes it difficult to continue and schedule blood drives. It’s just harder to meet our required quota numbers.”
Blood donations followed the wave of the pandemic, he said. Things got better, if not back to normal, during the summer months, only to rapidly drop off again in the fall.
“Now with the resurgence of COVID that really started in November, it’s been going on since,” he said, adding that blood drive cancellations have again increased, and that where some have gone ahead as planned, the number of donors is again down.
So, while January is always a good time for National Blood Donor Month, it is especially true this year.
“In December and January, historically, even without COVID, that’s always a low turnout period due to the holidays, people going on vacation, and due to the weather,” Corley said. “It just is a low time period for collections overall, and then overlay on top of that the third or fourth wave of COVID.”
There are other factors as well for the 21 military donor centers around the world.
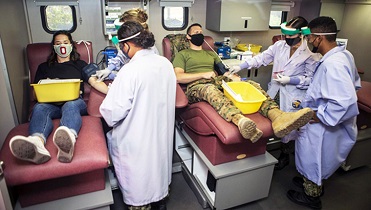 U.S. Navy corpsmen prep volunteers to donate blood and be tested for coronavirus antibodies outside of the Center for Naval Aviation Technical Training Marine Detachment at Camp Pendleton, California, in August 2020. The event was hosted by the Armed Services Blood Program. (Photo by Marine Lance Cpl. Drake Nickels.)
U.S. Navy corpsmen prep volunteers to donate blood and be tested for coronavirus antibodies outside of the Center for Naval Aviation Technical Training Marine Detachment at Camp Pendleton, California, in August 2020. The event was hosted by the Armed Services Blood Program. (Photo by Marine Lance Cpl. Drake Nickels.)
“With organizations going into and out of quarantine, service members being placed on ‘restriction of movement’ and changes in our health protection levels, it’s harder to schedule blood drives and make appointments for donors,” said Mark Salcedo, a blood donor recruiter with the Armed Services Blood Program (ASBP). “I was talking with a fellow recruiter and she reminded me of all the commands who have their staff teleworking.”
Salcedo said that when the ASBP cannot collect enough blood from donors, the blood bank must reach out to other military donor centers for blood, or even try to buy blood from the civilian market.
Collecting COVID-19 convalescent plasma meant blood collection during 2020 took on a new urgency. In April of last year, the FDA approved guidance for manufacture and transfusion of COVID-19 convalescent plasma, Corley explained.
“That has definitely been a product that the military and civilian blood industry has made in great numbers in order to support COVID patients,” he said. “Without a doubt, that blood product is having a great impact. For our standard blood products that we were already making, overall, there isn’t a large blood use for COVID patients — for red blood cells, or platelets, or for whole blood. But for COVID convalescent plasma, it has been approved by the FDA under an Emergency Use Authorization as a COVID treatment option.”
The convalescent plasma comes from recovered COVID-19 patients whose anti-body levels are at a certain level mandated by FDA, Corley said. Demand for that product has grown as the COVID-19 pandemic continues.
Air Force Tech. Sgt. Mark Friskel, an independent duty medical technician and flight chief at Arnold Air Force Base in Tennessee, has given blood regularly for the past eight years or so.
The process is “super easy,” he said, adding that he was not at all concerned about giving blood during the COVID-19 pandemic. “Basically, you lay down and relax watch a movie — you don’t have to do anything, really.”
But Friskel, a 14-year veteran, is concerned that information is lacking about blood donations and blood banks. He added that mentorship of new troops, such as during the First Term Airmen Course, would be an ideal time to explain the ease of blood donation and its value.
Friskel also suggested senior enlisted personnel getting the message out to fellow airmen over social media more often, or through individual videos aimed at his fellow airmen and women who have never donated before.
“I know when I was deployed, we needed blood all the time,” Friskel said.
These days, deployments are often domestic and include the Reserves and National Guard. And that’s another aspect of giving right now that’s a bit different for service members. Accustomed to protecting American citizens while abroad on the battlefield or on ships at sea, during times of COVID-19 that job can be much simpler with the humble act of a blood donation that could protect a civilian battling the virus in a hospital just down the street.
“Is there light at the end of the tunnel? Who knows? Is the vaccine going to solve our donor shortage? Only time will tell,” said Salcedo.
“I’ve been at this nearly 40 years both in and out of uniform,” Salcedo added. “I can say this has probably been one of the toughest years for the blood program and for many donor centers. But our leadership and our staff members continue to fight every day because they know the value in what we do for our health care mission.”