Malaria during the Korean War
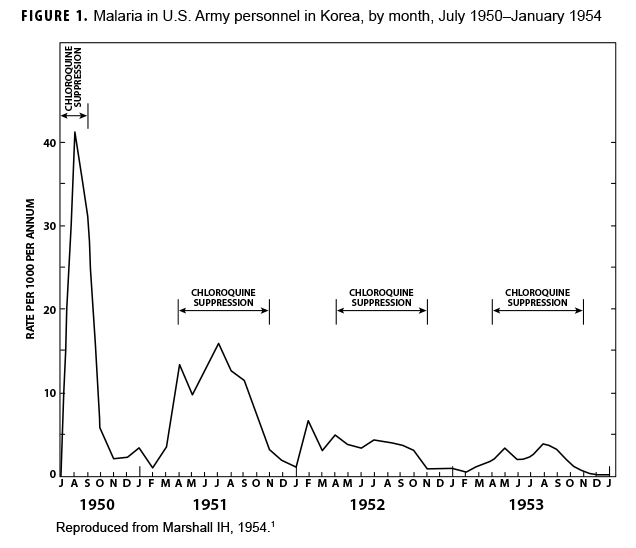
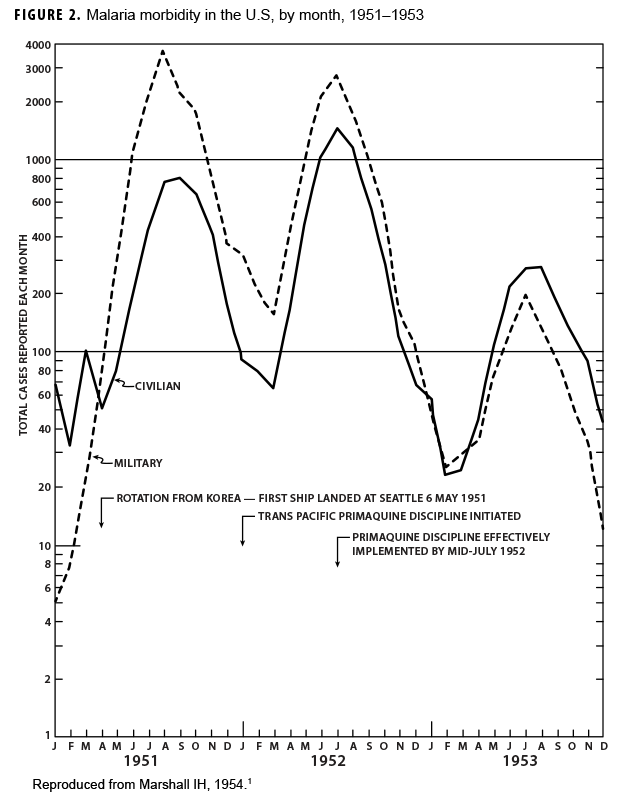
During the Korean War (1950–1953) malaria was a major infectious disease threat to infantry forces operating in Korea during the summer transmission season. Plasmodium vivax with a long latency period ad evolved such that many soldiers were exposed to infectious mosquitoes during their service in Korea during the summer but only became aware of their infection during the next year when latent hepatic parasites (hypnozoites) reactivated to cause symptomatic relapses. Chloroquine prophylaxis taken by soldiers during their time in the malarious region adequately suppressed any parasites in the blood minimizing the impact of malaria while in the combat zone (Figure 1) but did not kill hypnozoites. The result was many relapse cases long after exposure to the mosquito vectors.1 Due to 1-year tours of duty, most soldiers who contracted malaria during the Korean War were not actually symptomatic while in Korea. Thousands of cases of vivax malaria, mostly in soldiers, appeared in the U.S. beginning mid-year in 1951 (Figure 2), endangering the recently acquired national malaria elimination status. Clearly, better anti-malarial medication was required.
An 8-aminoquinoline, pamaquine, was the original synthetic antimalarial drug but it was judged by the U.S. Army to be too toxic for use because of its association with hemolysis in African American soldiers, many of whom were glucose-6-phosphate dehydrogenase (G6PD) deficient.2 A series of pamaquine analogues were tested by a reactivated antimalarial drug development program which had been initiated during World War II. Using prison volunteers purposely infected with rapidly relapsing vivax malaria strains from the Southwest Pacific, clinical investigators in Illinois rapidly identified a better tolerated 8-aminoquinoline known as primaquine.3,4 Once primaquine had been proven to kill hypnozoites in the liver, it was moved to field trials on troopships of returning Korean War veterans. By 1952, all troop transports had dedicated teams of medics whose function was to see that a 2-week course of primaquine (15 mg daily) was administered to every returning veteran. Within 2 years of implementing this strategy for post-exposure malaria prophylaxis, late vivax relapses in the U.S. had largely ceased due to the administration of primaquine to hundreds of thousands of soldiers.5 Chloroquine and primaquine remained the main antimalarial prophylaxis drugs even into the Vietnam conflict (1965–1972).
Tafenoquine
The emergence of drug-resistant malaria strains during the Vietnam conflict reinvigorated the drug development efforts by the U.S. Department of Defense (DOD) to combat this growing threat. During this effort, scientists from the Walter Reed Army Institute of Research, Division of Experimental Therapeutics (WRAIR/ET), screened thousands of potential new anti-relapse drug candidates to improve on the current standard of care, primaquine.6 During this testing one compound, called WR238605, or tafenoquine, demonstrated desirable properties that appeared superior to those of other pre-clinical candidates and primaquine, and became a lead candidate. Field trials for tafenoquine began in 1998.
After completing extensive pre-clinical and early clinical work, WRAIR/ET transitioned tafenoquine to the U.S. Army Medical Materiel Development Activity (USAMMDA). USAMMDA continued development of tafenoquine in collaboration with WRAIR/ET, its overseas laboratories, and through commercial partnerships, ultimately establishing a cooperative research and development agreement with 60 Degrees Pharmaceutical, LLC (60 Degrees). The partnership culminated in the U.S. Food and Drug Administration (FDA) approval of tafenoquine, (trade name Arakoda) in 2018 as an antimalarial indicated for the prophylaxis of malaria for continuous dosing up to six months in patients aged 18 years and older.7 Also in 2018, the FDA approved the use of tafenoquine (trade name Krintafel) for anti-relapse therapy of P. vivax in patients aged 16 years and older.8
Over the last decade, the DOD has seen relativity few cases of malaria, typically between 30 to 60 cases annually.9 Although cases of Plasmodium falciparum malaria acquired in Africa have become more common than cases caused by other species, the numbers of cases associated with service in South Korea and Afghanistan (almost exclusively P. vivax) have accounted for about 25% of the recent annual totals. The low case numbers are likely attributable to the reduced presence of U.S. Armed Forces in Afghanistan and Iraq in recent years, force health protection (FHP) measures to counter the threat from the mosquito vectors, such as permethrin treated uniforms and bed nets, and command discipline to ensure service members take their chemoprophylaxis and wear uniforms properly. Tafenoquine will likely have a significant role in reducing the number of malaria cases further by increasing compliance, where weekly dosing could be preferred over daily dosing.10
The promise of tafenoquine is based upon several characteristics. First, tafenoquine is effective against all species and life cycle stages of the malaria parasites that infect humans; at this time, there is no known tafenoquine resistance among the 5 Plasmodium species that affect humans. Second, the drug is FDA-approved for up to 6 months of malaria prophylaxis while living or traveling in a malaria region. Third, the effective half-life of the drug in humans is at least 2 weeks. As a result, the frequency of maintenance doses is just weekly. This dosing schedule enhances the likelihood of good compliance, particularly in settings where supervised or observed dosing is desirable, such as in military units. The drug's long half-life provides sufficiently high drug levels to allow for what is called "compliance forgiveness". If a service member misses a weekly dose, there is enough drug remaining in the body to provide protective efficacy until the following scheduled dose. Although it is not recommended to miss a weekly dose, the label instructions specify that, when a weekly dose is omitted, the individual should not take a make-up dose but should simply resume the prophylaxis at the time of the next scheduled dose. Results of clinical trials have suggested that monthly dosing could be a possibility in the future.7,11 Fourth, not only is tafenoquine effective for anti-relapse therapy (post-exposure prophylaxis) against the hypnozoites of P. vivax and Plasmodium ovale, but such therapy requires just a single dose of tafenoquine. This single dose requirement contrasts with the conventional dose schedule of primaquine which must be taken daily for 14 days, a well-known impediment to high levels of patient compliance.12 Moreover, if the weekly prophylaxis while in the malarious area consisted of tafenoquine, no additional anti-relapse therapy would be required.
In December 2019, the Defense Health Agency (DHA) published an update to Deployment Health Procedures, procedural instruction (PI) 6490.03 approving tafenoquine as a second-line malaria prophylaxis countermeasure for FHP.13 This update is the first step in the introduction of tafenoquine to the warfighter. Combatant Commands, such as U.S. Africa Command (AFRICOM) and U.S. Indo-Pacific Command (INDOPACOM) have applied the DHA PI updates and incorporated tafenoquine as a new malaria prophylaxis option in their internal policies. As the drug is administered in the broader military and civilian population and 60 Degrees completes the FDA post-marketing commitments, more information on, and familiarization with, the properties of the drug will be realized and it is expected that DOD's guidance will evolve to integrate the new information.
As noted in PI 6490.03, there are additional factors to consider when contemplating the use of tafenoquine.13 First, tafenoquine (as well as primaquine) should not be prescribed for persons who have G6PD deficiency because of the risk of drug induced hemolytic anemia. Current DOD policy provides for the routine screening of all service members for G6PD deficiency and for documentation of the results in the service members' individual health records. Second, current FDA approval of tafenoquine for chemoprophylaxis specifies a duration of use of no more than 6 months; however, there are ongoing post-marketing studies to extend the duration of use to 12 months.14
Editorial Comments
Malaria relapses are an adaptation of the parasite to survive between transmission seasons through latency in the liver followed by reactivation months to even a year after infection. Many U.S. Army veterans who served in the Southwest Pacific during the World War II reported greater than 20 separate malaria episodes triggered by relapses from the liver despite taking chemo-suppressive medications. Pamaquine was too toxic for use but its better tolerated cousin primaquine largely solved the problem of post-deployment relapses during the Korean War. Efficacious medications are only part of the equation needed for force health protection. Better tolerated drugs that could be given infrequently enough (e.g., weekly as opposed to daily) so as to facilitate supervised administration of the medication (directly observed therapy) are also desirable.
Despite a very long developmental history, tafenoquine is now available to replace primaquine as a better tolerated medication to treat soldiers infected with relapsing malaria.14 In addition, the very long (2-week) half-life of tafenoquine allows it to be given weekly (200mg in adults) for reliable chemoprophylaxis following a 3-day loading dose regimen consisting of 200 mg per day for a total of 600 mg. It seems likely, based on work in the Royal Thai Army, that tafenoquine monthly regimens may eventually be devised which would further increase compliance and thus effectiveness.11 Anti-relapse therapy consists of a single dose of 300 mg of tafenoquine taken after departure from the area of malaria risk. Further work at WRAIR/ET is being conducted with the aim of finding a regimen or combination that can be safely given to G6PD-deficient individuals, but currently tafenoquine is limited to those known to have adequate G6PD activity by laboratory measurement. Tafenoquine is effective against all malaria species and life cycle stages of the malaria parasite that infect humans, has no known malaria resistance, and provides a convenient dosing regimen, all of which will likely result in vastly improved compliance and effectiveness in the prevention of malaria in U.S. service members. Whether tafenoquine will have a major role in public health efforts to eliminate malaria globally remains to be seen, but tafenoquine is certainly a major advance in FHP against malaria for soldiers deployed to endemic areas.
Author affiliations: U.S. Army Medical Materiel Development Activity (MAJ Zottig); Australian Defence Force Malaria and Infectious Diseases Institute, Enoggera, QLD, Australia (Dr. Shanks).
Acknowledgements: The authors thank all those who worked to bring tafenoquine to registration for malaria chemoprophylaxis and treatment.
Disclaimer: The opinions expressed are those of the authors and do not necessarily reflect those of the Australian Defence Force or the U.S. Department of Defense. Discussion of specific pharmaceutical products does not reflect an endorsement of those products.
Conflict of interest: The authors do not claim any conflict of interest.
Funding: Authors are employees of the U.S. Department of Defense or the Australian Defence Organization. No specific funding was given for this historical study.
References
- Marshall IH. Malaria in Korea. In: Recent Advances in Medicine and Surgery Based on Professional Medical Experiences in Japan and Korea 1950–1953. Vol 2. Washington, DC: US Army;1954:270–283. Accessed 13 August 2020. https://history.amedd.army.mil/booksdocs/korea/recad2/recadvol2.html
- Hockwald RS, Arnold J, Clayman CB, Alving AS. Toxicity of primaquine in Negroes. J Am Med Assoc. 1952;149(17):1568–1570.
- Alving AS, Arnold J, Robinson DH. Mass therapy of subclinical vivax malaria with primaquine. J Am Med Assoc. 1952;149(17):1558–1562.
- Garrison PL, Hankey DD, Coker WG, et al. Cure of Korean vivax malaria with pamaquine and primaquine. J Am Med Assoc. 1952;149(17):1562–1563.
- Archambeault CP. Mass antimalarial therapy in veterans returning from Korea. J Am Med Assoc. 1954;154(17):1411–1415.
- Milhous WK, Schuster BG. Malaria studies aim at drug resistance. US Med. 1990;26:27–28.
- U.S. Food & Drug Administration Administration. Drugs@FDA: FDA-Approved Drugs: ARAKODATM. Accessed 16 November 2020. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=210607
- U.S. Food & Drug Administration Administration. Drugs@FDA: FDA-Approved Drugs: KRINTAFELTM. Accessed 16 November 2020. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=210795
- Armed Forces Health Surveillance Branch. Update:Malaria, U.S. Armed Forces, 2019. MSMR.2020;27(2):2–7.
- Tan KR, Magill AJ, Parise ME, Arguin PM. Doxycycline for malaria chemoprophylaxis and treatment: Report from the CDC expert meeting on malaria chemoprophylaxis. Am J Trop Med Hyg 2011; 84(4):517–531.
- Walsh DS, Eamsila C, Sasiprapha T, et al. Efficacy of monthly tafenoquine for prophylaxis of Plasmodium vivax and multidrug-resistant P. falciparum malaria. J Infect Dis. 2004;190(8):1456–1463.
- Kotwal RS, Wenzel RB, Sterling RA, et al. An outbreak of malaria in US Army Rangers returning from Afghanistan. JAMA. 2005; 293(2):212–216. Erratum in: JAMA. 2005: 293(6):678.
- Defense Health Agency. Procedural Instruction 6490.03. Deployment Health Procedures. 17 December 2019.
- Tan KR, Hwang J. Tafenoquine receives regulatory approval in USA for prophylaxis of malaria and radical cure of Plasmodium vivax. J Travel Med. 2018;25(1).