Skip subpage navigation
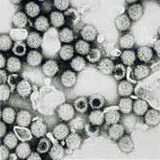
Rotavirus is the most common cause of severe gastroenteritis in infants and young children worldwide. Before initiation of the immunization program in 2006, nearly every child in the United States was infected with rotavirus by age five years. Severe dehydrating gastroenteritis caused by rotavirus occurred primarily among children aged 4-23 months. Rotavirus illness in children ranges from mild, watery diarrhea of limited duration to severe diarrhea with vomiting and fever than can result in dehydration with shock, electrolyte imbalance, and death.
There are two rotavirus vaccines licensed for use in the U.S. Both are administered orally; one is given in a 3-dose series at ages 2, 4, and 6 months, and the other in a 2-dose series at ages 2 and 4 months.
The minimum age for dose one of rotavirus vaccine is 6 weeks; the maximum age for dose 1 is 14 weeks and 6 days. Vaccination should not be initiated for infants aged 15 weeks and 0 days or older because of insufficient data on safety of dose one of rotavirus vaccine in older infants. The minimum interval between doses of rotavirus vaccine is 4 weeks. All doses should be administered by age 8 months and 0 days.
Source: Centers for Disease Control and Prevention
You will find below all of the resources you will need about the rotavirus vaccine. More will be added as they are published or released.
You also may be interested in...
Fact Sheet
Feb 16, 2024
 .PDF |
86.25 KB
.PDF |
86.25 KB
This information paper describes Rotavirus disease and vaccine to prevent it
You are leaving Health.mil
The appearance of hyperlinks does not constitute endorsement by the Department of Defense of non-U.S. Government sites or the information, products, or services contained therein. Although the Defense Health Agency may or may not use these sites as additional distribution channels for Department of Defense information, it does not exercise editorial control over all of the information that you may find at these locations. Such links are provided consistent with the stated purpose of this website.
You are leaving Health.mil
View the external links disclaimer.
Last Updated: May 14, 2024